Though early cancer diagnosis provides better outcomes for patients, many experience later disease recurrence following surgery to remove the primary tumor. This is due to a small number of cancer cells that were not removed during surgery, referred to as minimal residual disease (MRD). To address this, adjuvant chemotherapy is often used post-surgery to eliminate these residual cancer cells on a ‘just in case’ basis, which is typically based on the tumor characteristics at the time of surgery and not the patient’s disease status after surgery.
In a new article in Nature’s Biopharma Dealmakers journal, Joshua Cohen, a clinical researcher at Johns Hopkins University School of Medicine, underscored the issue of adjuvant chemotherapy decisions for colorectal cancer patients, “To avoid the risk of recurrent disease, we often overtreat patients with stage 2 colorectal cancer based on pathological and clinical criteria and physician choice. The clinical benefit for adjuvant therapy for these patients is modest. In stage 3 patients, the benefit of adjuvant treatment is undisputed, but we still need to know how aggressively these patients should be treated, as many receive therapy they don’t need and some don’t receive enough or the appropriate type.”
Earlier this year, the pivotal DYNAMIC trial demonstrated the clinical benefit of using ctDNA MRD testing for informing adjuvant therapy decisions for patients with stage 2 colorectal cancers. “This was a paradigm-changing clinical trial. It showed that physicians could reduce adjuvant chemotherapy use without compromising survival,” said Peter Gibbs, Professor of Medicine at The Walter and Eliza Hall Institute (WEHI) of Medical Research in Melbourne.
Haystack’s technology is an evolution of the chemistry used for ctDNA detection in the DYNAMIC trial. Haystack offers more sensitive chemistry for even greater detection capabilities, eliminating unnecessary adjuvant therapy and ensuring that all patients that would benefit from adjuvant chemotherapy receive it. Compared to a single biopsy at the time of surgery, ctDNA testing allows for continued MRD surveillance with a relatively non-invasive blood test. Beyond MRD, the same technology can help physicians select the best therapy regimen and monitor treatment responses.
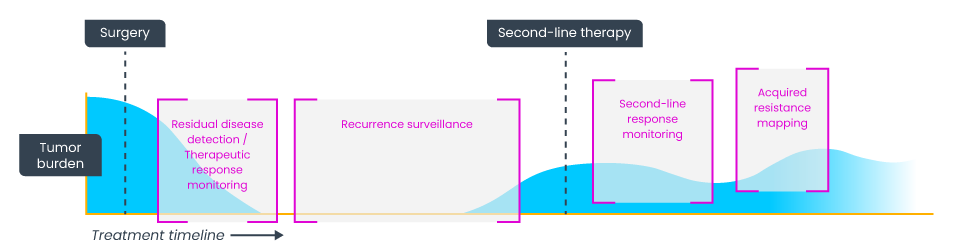
Haystack’s technology can detect as few as one mutation in a million DNA molecules, which positions it as the most sensitive ctDNA-based MRD test for use in solid tumors. Ultrasensitive MRD testing such as Haystack’s can optimize cancer clinical trials and help clinicians make informed, personalized decisions to improve patient outcomes.
To learn more about how we’re working to improve patient outcomes by applying precision technology to detect residual disease, read the full article in Nature Biopharma Dealmakers here.


