Haystack Oncology will be attending the 2023 European Society for Medical Oncology Congress (ESMO 2023) taking place in Madrid, Spain from October 20-24.
Last year’s ESMO drew nearly 30,000 medical oncology professionals from 152 countries. The annual conference aims to further medical oncology science and to improve the lives of patients through continued education.
About Haystack MRD™
Haystack MRD™ is a tumor-informed ctDNA detection test for postsurgical measurement of residual and recurrent disease in solid tumor settings. Powered by Haystack’s technology, the test significantly reduces noise to detect exceedingly low numbers of ctDNA molecules in blood. Our unique technology translates to improved minimal residual disease (MRD) detection and more confident clinical decision-making.
With the addition of Haystack MRD, Quest’s cancer care portfolio includes unmatched prognostic and monitoring capabilities in MRD testing. Quest is a single-source solution for guideline-driven cancer care.
Why choose Haystack MRD?
Ultrahigh sensitivity for detection of:
- MRD
- Therapeutic response monitoring
- Surveillance for cancer recurrence
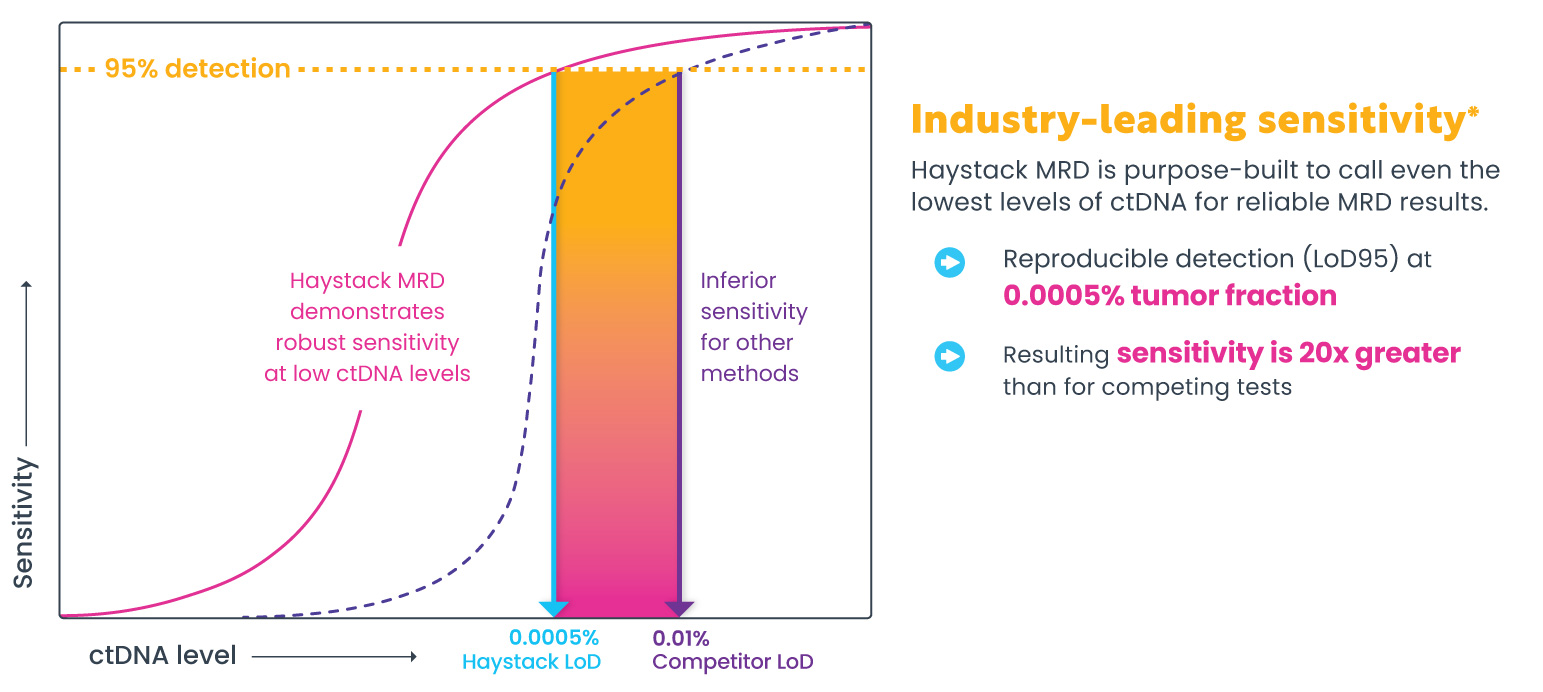
Haystack MRD delivers industry-leading sensitivity with the ability to detect 1 mutant DNA molecule in 1 million normal DNA molecules.1 As a result, extremely low levels of ctDNA can be called with confidence. Learn more about Haystack MRD’s exquisite sensitivity.
Tumor-informed, personalized assay
A personalized assay for each patient is developed based on “ground-truth” genetic variations identified via whole-exome sequencing (WES) of the original tumor sample. Learn more about the benefits of tumor-informed MRD testing.
Demonstrated clinical utility
For the first time, data are available that support using ctDNA MRD testing to guide adjuvant therapy decisions. Researchers used an earlier version of Haystack’s technology in the DYNAMIC study to demonstrate the clinical benefit of MRD testing in stage II colon cancer patients.
Accelerate clinical development
Haystack MRD leverages ultrahigh sensitivity and specificity to produce insights that can drive biopharma pipeline development. With industry-leading sensitivity, Haystack MRD identifies 2 times more patients with residual disease than competing tests do, enabling optimization of patient selection and faster trial completion.1 Learn more about how Haystack MRD can support clinical development.
Built on decades of ctDNA clinical technology development
Haystack MRD was built from the ground up by pioneers in liquid biopsy technology to overcome the unique challenges of ctDNA detection in the MRD setting. It does not rely on methods adapted from other intended uses such as NIPT (noninvasive prenatal testing) and tumor profiling of metastatic cancers.
Learn more at ESMO 2023
To connect at ESMO, stop by Booth 586 or get in touch today to schedule a meeting. Ask us about our clinical data.
1Data on file. Haystack Oncology; 2023.



